i-Fect used In Vivo for Study
Researchers use our
i-FectTM to effectively deliver miR-126
in vivo to modulate Methyl-CpG-binding protein 2 (MeCP2).
MeCP2 regulates gene expression through activation, repression and chromatin remodeling. Mutations in MeCP2 cause Rett syndrome, and these patients display impaired nociception.
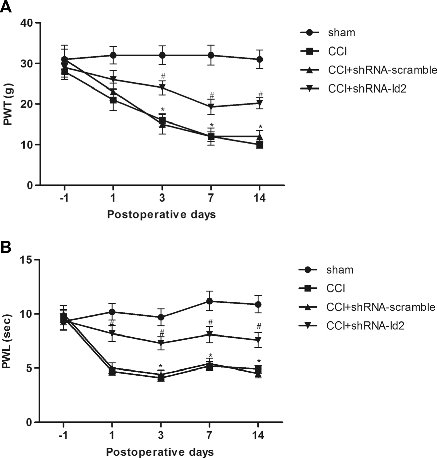
The researchers observed an increase in MeCP2 expression in mouse dorsal root ganglia (DRG) after peripheral nerve injury: Melissa T. Manners, Adam Ertel, Yuzhen Tian and Seena K. Ajit. Genome-wide redistribution of MeCP2 in dorsal root ganglia after peripheral nerve injury. Epigenetics & Chromatin 20169:23. DOI: 10.1186/s13072-016-0073-5© The Author(s) 2016 Received: 11 March 2016. Accepted: 27 May 2016Published: 7 June 2016...miRNA administration protocol was adapted from previous report of intrathecal miRNA delivery. To administer miRNA mimics, a polyurethane catheter (25G, 5.5 cm long, SAI infusion) was placed into the intrathecal space of the lumber L4–L5 vertebrae under isoflurane anesthesia. The catheter was stereotactically secured under the skin and occluded between injections. A custom miRCURY (Exiqon) miR-126 mimic containing a 5′ cholesterol tag and 3′ fluorescein label was injected at 2 nmol concentration with 4 µl
iFECT transfection reagent (Neuromics). A total of 6 µl was delivered into the catheter connection juncture using a 25G blunt end needle on a Hamilton syringe. The catheter was then flushed with 7 µl sterile PBS to ensure miRNA reached the intrathecal space...

Figure: Expression of miR-126 and its target genes Dnmt1 and Vegfa in the DRG after nerve injury. a Relative expression of miR-126 determined by qPCR shows a reduction in miR-126 in SNI model compared to DRG from sham control. U6 was used for normalization (n = 8 sham, n = 7 SNI). b Relative expression of Dnmt1 mRNA and c Vegfa transcripts showed an increase in the DRG after nerve injury compared to control (n = 3). Gapdh was used as a normalizer. d Representative Western blot and quantification showed an increase of Dnmt1 protein in the DRG after nerve injury. e Western blot and quantification showed Vegfa protein was not significantly different in DRG after nerve injury (n = 3 from pooled samples, three DRG were pooled for each sample).
Conclusions: The study shows a regulatory role for MeCP2 in that changes in global redistribution can result in direct and indirect modulation of gene expression in the DRG. Alterations in genome-wide binding of MeCP2 therefore provide a molecular basis for a better understanding of epigenetic regulation-induced molecular changes underlying nerve injury.