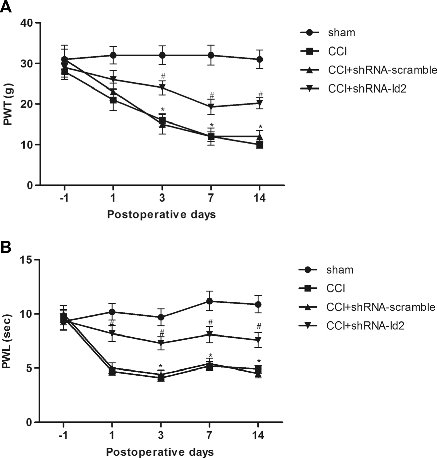
Our i-FectTM Transfection Kit is used to study Epigenetics and pain. Here's yet another example: M. Leinders, b, N. Üçeyler, R.A. Pritchard, C. Sommer, L.S. Sorkin. Increased miR-132-3p expression is associated with chronic neuropathic pain. Experimental Neurology. Volume 283, Part A, September 2016, Pages 276–286...The inhibitor and mimetic were administered to awake rats via the it catheters. Prior to injection, active or mismatch inhibitors were mixed with (1:5 w/v) i-Fect™ in vivo transfection reagent(Neuromics, Edina, USA) to final doses of 5, 2 and 1 μg in 10 μl...
.
Spinal administration of miR-132-3p antagonists via intrathecal (i.t.) catheters dose dependently reversed mechanical allodyina and eliminated pain behavior in the place escape avoidance paradigm (p < 0.001). Intrathecal administration of miR-132-3p mimetic dose-dependently induced pain behavior in naïve rats (p < 0.001). Taken together these results indicate a pro-nociceptive effect of miR-132-3p in chronic neuropathic pain.Finding like these could pave the way for an miRNA like therapy for pain.